If you have registration duties under REACH, then you need to know what you should be doing and by when. This information is a starting point to help you understand what you need to do to fulfil these duties.
What is registration?
Registration requires manufacturers, importers and Only Representatives (ORs) – registrants, to collate information on the substances they manufacture or import. Where appropriate, they use that information to assess any potential hazards. With this information on the properties of a substance, and by working with others in the supply chain, the registrant can, when appropriate, assess any risks to human health and/or the environment and develop appropriate risk management measures for the various uses of a substance.
This information is sent to the European Chemicals Agency (ECHA) as an electronic summary file (a registration dossier).
Steps to Registration
1. Data-sharing
One of the key objectives in REACH is to gain information about the properties of substances being manufactured, supplied and used in the EU. However, this should be achieved by using the minimum number of animal tests. One of the main ways to reduce the amount of animal testing is through the mandatory sharing of animal test data. Depending on the type of substance (phase-in or non-phase-in) and whether it has been pre-registered (where applicable), this data-sharing is achieved through one of two mechanisms: Substance Information Exchange Forums (SIEFs) or Article 26 Inquiry.
The Substance Information Exchange Forum (SIEF)
This data-sharing mechanism is used for phase-in substances that have been pre-registered. All the companies that pre-register a substance will be members of the Substance Information Exchange Forum (SIEF) for that substance and can benefit from the phased registration deadlines. Further information on pre-registration and the registration deadlines is available is available at the following link:
The members of the SIEF should communicate with each other, pool the existing information available to them and decide on how to fill any information gaps. The UK REACH CA cannot be actively involved with this process.
For a SIEF to operate effectively somebody will need to coordinate its activity and initiate communications, organise meetings, etc. One or more members of the SIEF may indicate that they wish to be the SIEF Formation Facilitator (SFF). This is not a formal role in REACH, but may offer a way to get things started. Once the SIEF is active, a 'Lead Registrant' can be identified. The SFF does not need to be the same as the Lead Registrant.
The Lead Registrant's role is to produce the 'Lead dossier' to which the other joint registrants will refer. That is, the Lead Registrant will submit a complete dossier containing all the required data for physicochemical, toxicological end environmental properties.
Joint registrants submit 'partial dossiers' containing information specific to their company; for example, information about their identified uses, their production volumes or their 'version' of the substance (e.g., to account for different impurities). In these joint submissions, instead of test summaries, they simply refer back to the lead dossier.
It is possible to appoint a Third Party Representative (TPR) to represent you in the SIEF. This can be for commercial confidentiality reasons or to provide expertise in negotiating the data-share. However, the TPR does not register on your behalf and you retain legal liability for the registration.
The costs associated with performing tests, gaining access to test summaries, running the SIEF, etc are decided upon by the members of the SIEF. More detailed guidance SIEFs is in the ECHA guidance on data-sharing.
Article 26 Inquiry
This data-sharing mechanism is used for non phase-in substances or for phase-in substances which have not been pre-registered; both of which must be registered for supply to start or continue (respectively). Before any registration is submitted, an inquiry must first be sent to ECHA to determine whether there has been a previous registration or inquiry for the same substance. This is required under Article 26 of the regulations and is done via the REACH-IT System operated by ECHA.
The outcome of the inquiry will depend on whether the substance was previously registered, or if others are also interested in registration. The possible outcomes are summarised in the Article 26 Inquiry flow chart below:
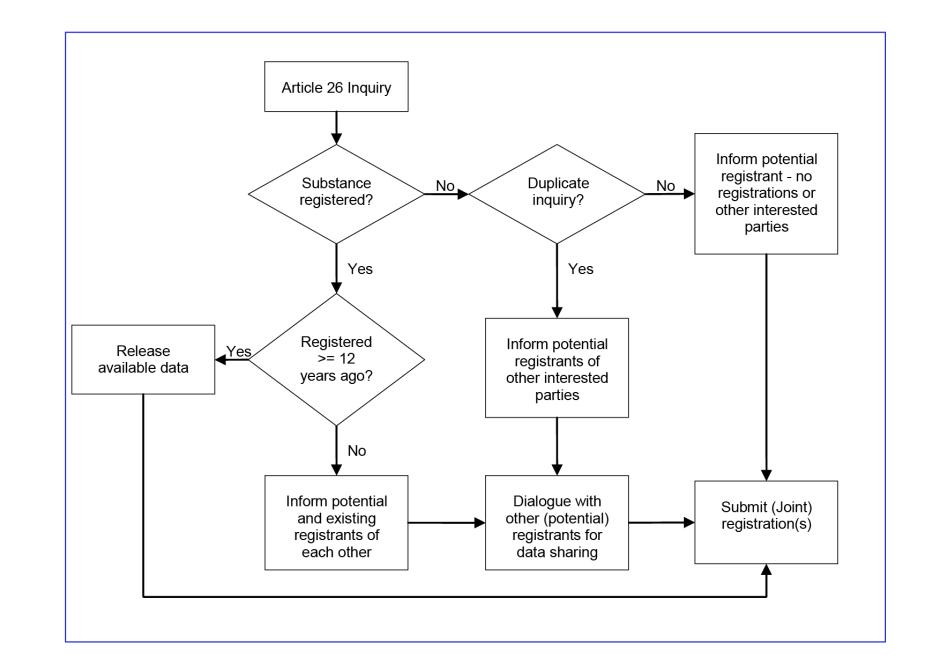
If there is an existing registration, then ECHA can release any physico-chemical, toxicological or environmental data that they have held for 12 years or more. For substances 'transferred' into REACH from the old NONS scheme, the 12 years is from the time of acceptance of the data under the NONS scheme. To access data held for less time, potential registrants must seek permission from the original data holder (usually a previous registrant).
If duplicate inquiries are made, then ECHA will put the potential registrants in touch with each other so that a joint submission can be made.
When, due to a failure to pre-register, a duty holder has had to register a substance in order for manufacture/supply to continue, then following registration they will be added to the existing SIEF for that substance. This allows other potential registrants to use the information that this 'early registrant' has submitted to ECHA.
More information on the inquiry process is available in the ECHA Guidance on Data-sharing.
2. Information requirements
There are two main components to the registration dossier, a technical dossier and a chemical safety report (CSR). A CSR is only required for substances registered in the 10 tonne/annum tonnage band or above.
For the technical dossier, registrants should collect all the relevant information available already. This information should then be compared against the information requirements outlined in Annexes VI to X of REACH, which specify the information required for the different tonnage bands.
For substances being supplied in higher tonnage bands, the requirements are cumulative; for example, in the 10 tonnes or more per annum band, you need the data specified in Annexes VI, VII & VIII; at 100 tonnes or more, Annexes VI, VII, VIII and IX, etc. If all the information required is not available then any registrants needing information should, with other potential registrants, decide upon the best way to fulfil this data gap. Registrants are required only to submit (or have access to) the information relevant to their tonnage band.
You should remember that it is not always necessary to fulfil an information requirement by conducting tests that use animals. There may be other options, such as in-vitro tests, using data from similar substances (known as read-across), using predicted properties/values obtained from models that look at chemical structure [known as Quantitative/Qualitative Structure-Activity Relationships (QSAR) or Quantitative Structure Property Relationships (QSPR)], or using arguments for not conducting a test based on properties (e.g., extreme pH), exposure, or a ‘Weight-of-Evidence’ approach.
All of these techniques are explained more in Annex XI of the REACH regulations and in the “Guidance on Information Requirements and Chemical Safety Assessment” (available of the ECHA website). If there is more than one registrant, you will need to agree on the cost and conduct of any testing deemed necessary.
In the case that testing is required, then for information required by Annexes VII or VIII you should generate this new information. For information requirements from Annexes IX or X (for the higher volume substances), then you should submit a testing proposal as part of your registration dossier and ECHA will consider this proposal. Testing should only start once the testing plan is approved.
The second part of the registration is the chemical safety report (CSR). This is the document which results from the chemical safety assessment (CSA). It can be submitted separately or as part of a joint submission covering all uses. For the CSR you will need information on the manufacturing processes (for substances made in the EU) and identified uses of the substance. Identified uses include your own use, those that you market a substance for and those that are made known to you by a downstream user.
To explore uses you should communicate with your downstream users. However, they are not obliged to let you know about their uses. For more information on Chemicals Safety Reports see the ECHA Guidance on information requirements and chemical safety assessment, especially Parts A, D, E & F.
3. Submitting your registration
The technical dossier must be compiled using the IUCLID software (or alternatively within REACH-IT for a joint submission). This is available to download (free of charge) from the IUCLID website:
Details of how to install and use the software are available on the IUCLID website. You should use the appropriate structured template in IUCLID for the tonnage band involved. If the tonnage of your substance is 10 tonnes per year or greater, and a chemical safety report is required, this should be a stand-alone document, which you should attach to the IUCLID dossier.
All dossier submissions and correspondence regarding the registration will occur via your REACH-IT account. More information is also available on the Registration section of “Dossier Submission Tools” part of the ECHA website.
4. What happens next?
Once ECHA receives your registration dossier, they will assign a submission number and communicate this to you. This is just an acknowledgement number and is NOT your registration number. It should be used as a reference in all correspondence relating to this registration until a registration number is assigned.
ECHA will then check the dossier for completeness. Part of this is an automated check to ensure that all the required sections have been completed. The second part is a check for financial completeness to ensure the appropriate fees have been paid on time. REACH registration fees are payable to ECHA and the precise fee payable depends on the registration tonnage band and company size (for details see the REACH Fees Regulations).
These fees do not include any costs associated with data acquisition or sharing. At least 5% of all registrations will also be selected to undergo a full compliance check. This is a rigorous check that the information required is present, scientifically valid and of the appropriate quality.
Within three weeks of the submission date ECHA will either contact you to inform you that the dossier is not complete or issue a registration number. For phase-in substances, manufacture or import can continue during this three week assessment period. For non-phase-in substances, manufacture or import (at > one tonne/annum) can start once this three week assessment period has ended.
For phase-in substances submitted near the deadline, this assessment period is extended to three months. If ECHA do not contact you during the three week or three month period (as appropriate), then you can assume the dossier is complete.
If ECHA decide your dossier is not complete, you will be sent a report indicating the missing information and giving a deadline for the submission of this additional information. You should then add this information to the dossier and resubmit the entire dossier to ECHA before the set deadline. A new submission date will be assigned and a new completeness check will be done.
If it is again found to be lacking information, ECHA will reject the registration and you will risk losing your fee. Manufacture or import of this substance by the applicant is then not permitted within the EU. If you still wish to register the substance, you will need to start from the beginning of the process, possibly including paying the fee again.
Once the registration is deemed complete, it will be automatically assigned a registration number and this will be sent to you by ECHA without delay. The date of registration will be the same as the latest submission date. You should now use this registration number in all further correspondence relating to this registration. The competent authority of the member state in which the registrant is based will be notified of the acceptance of the registration by ECHA.
5. Updating a registration
Once you have a registration in place for your substance, it is your responsibility to ensure that it is kept up to date.
You may wish to update your registration for various reasons, such as your substance composition has changed, tonnage band has increased, because you have additional information (e.g., about classification & labelling) or because the company information has changed. This new information should be submitted to ECHA without undue delay. For some types of update a fee is charged.
You may also need to update your registration because of a decision that has been made by ECHA; for example, following the submission of testing proposals. In this case, ECHA will issue a deadline for completion of the update.
You will also be informed by ECHA if a new registrant for your substance submits new information, you will then be expected to consider this information and update your registration if appropriate.
When submitting an update, this should also be done via REACH-IT on the ECHA website. You should submit the entire dossier again, including all the new information in the appropriate sections.